
![]()
Selective adsorption of bilirubin and bile acid from plasma.
No need for the replacement of plasma, minimizing the risk of infection with hepatitis, AIDS, etc.
Applicable to patients with protein allergy.
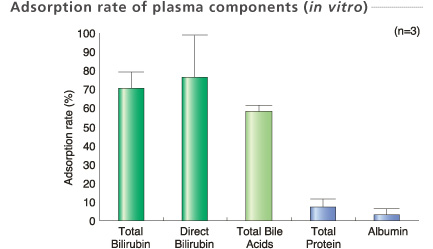
Plasma sample were collected at the column outlet point when 2.4 L (equivalent volume) plasma was treated.
Experimental conditions
Perfusion method: One-pass operation with small scale column (1/350)
Sample : Human plasma 2.4 L (Equivalent volume)
Flow rate : 20 mL/min (Equivalent rate)
In-house data
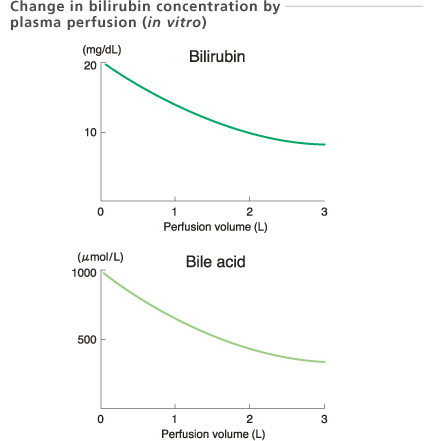
Experimental conditions
Sample : Bovine plasma 2 L (5 units/mL heparin, 20 mg/dL bilirubin and 1000 μ mol/L bile acid added bovine plasma)
Temperature : 37 ℃
Perfusion rate: 20 mL/min
In-house data
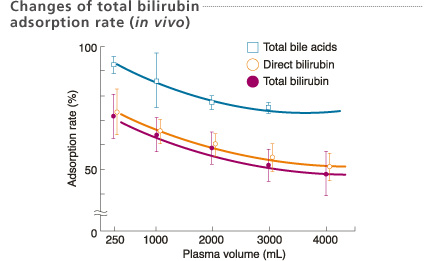
Patients : n=6
Total treatments : 29 times
First Department of Surgery, Okayama University, Japan
| Adsorbent | Material | Styrene divinylbenzene copolymer |
|---|---|---|
| Volume | 350 mL | |
| Priming Volume | 130 mL | |
| Container | Material | Polypropylene |
| Dimension | 220mm[L] x 62mm[D] | |
| Weight | 600 g | |
| Sterilization | High pressure steam | |
| Filter | Material | Polyethylene (coated with ethylene-vinylalcohol copolymer) |
|---|---|---|
| Area | 0.07 m2 | |
| Container | Material | Poly (vinyl chloride) |
| Dimension | 165mm[L] x 22mm[D] | |
| Priming Volume | 30 mL | |
| Sterilization | Ethylene oxide | |
The PLASORBA BR-350 is intended for the treatment of plasma. Never run whole blood through the PLASORBA BR-350. Thrombocytes cannot pass through the PLASORBA BR-350 and may cause blockage. Do not use PLASORBA BR-35 with plasma containing a large amount of thrombocytes.